
Note how large this peak is, easily the biggest in the spectrum. In general, for saturated ketones this peak appears at 1715 ± 10. The carbonyl stretch of acetone falls at 1716, and is labeled A in Figure 5. Note that the carbons attached to the carbonyl carbon are denoted “alpha carbons.”Ī common example of a ketone-containing molecule is acetone (dimethyl ketone), whose IR spectrum is shown in Figure 5. Ketones consist of a C=O with two carbons attached, one to the left and one to the right as illustrated in Figure 4.
Perhaps one of the most common chemical structures to contain a carbonyl group are ketone molecules. Thus, for almost every carbonyl containing functional group we discuss I will quote two carbonyl stretching regions, one for the saturated and one for the aromatic versions of the functional group. Conjugation weakens the C=O bond, lowers its force constant, and hence its peak position is lowered, on average about 30 cm -1 (1). The orbitals are close enough that they somewhat overlap, allowing some of the electron density from the carbonyl bond to be pulled off into the benzene ring in a phenomenon known as conjugation, which is illustrated at right in Figure 3. The carbonyl group also contains a p-type orbital that points through space toward the orbitals on the aromatic ring, Figure 3. This is because aromatic rings, such as benzene, contain p-type orbitals with electron density that sticks up out of the plane of the molecule (2) as illustrated to the left in Figure 3.
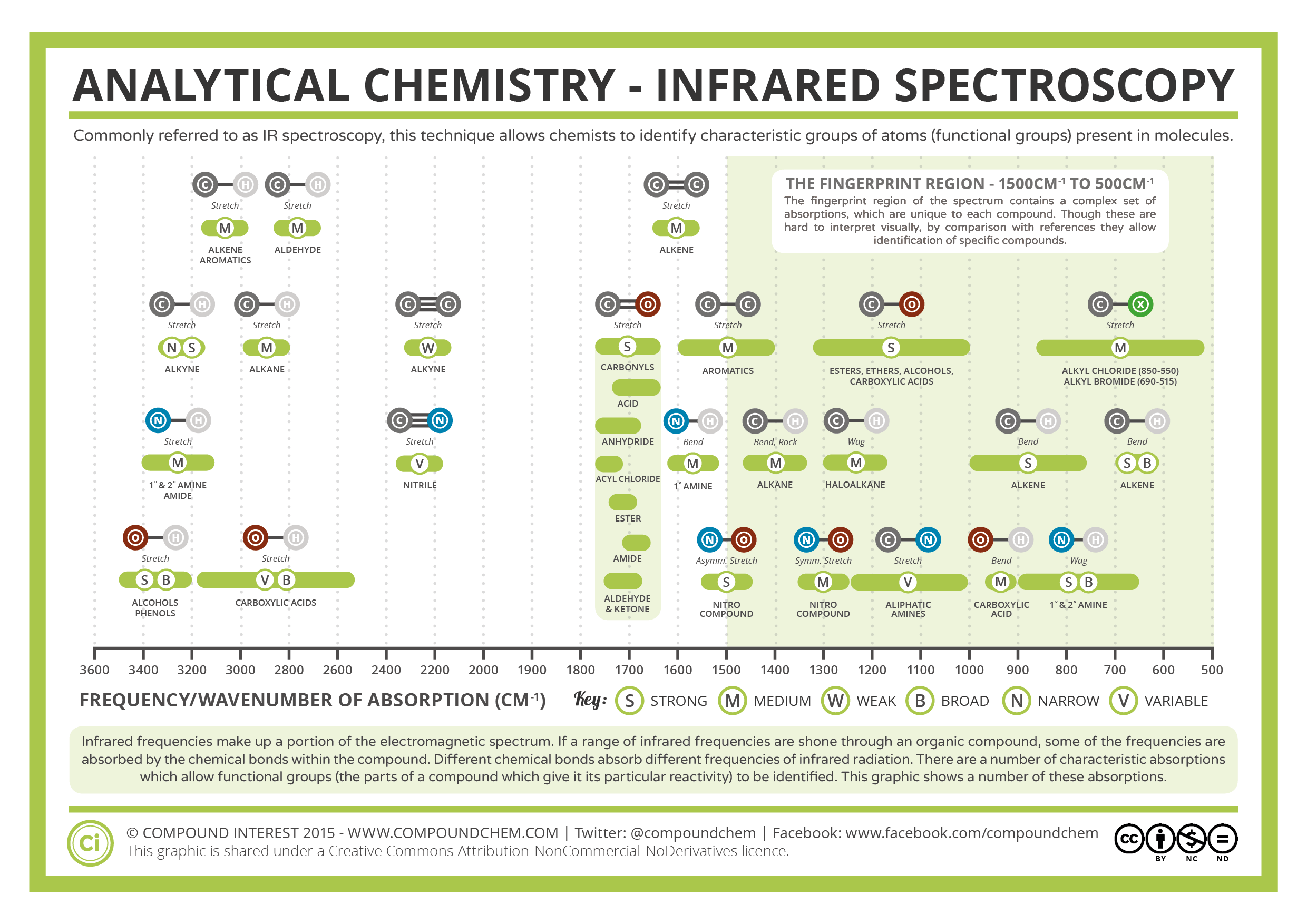
In general, aromatic C=O stretching peaks fall ~30 cm -1 lower than saturated C=O stretching peaks. Saturated and aromatic carbonyl groups can be distinguished from each other using IR spectroscopy. An example of an aromatic carbonyl group is shown in Figure 3. An aromatic carbonyl group has one or more aromatic carbons attached to the carbonyl carbon. A saturated carbonyl group has two saturated carbons attached to the carbonyl carbon. Because of this trait, an IR spectrometer is, in some respects, the perfect carbonyl detector.Ĭarbonyl bonds can be divided into two classes depending on what type of carbons are attached to the carbonyl carbon. This attribute makes C=O stretches one of the easiest IR peaks to spot and assign. The carbonyl stretching peak is perhaps the perfect example of a group wavenumber, a peak that shows up intensely in a unique and relatively narrow wavenumber range. This area is sometimes referred to as the carbonyl stretching region as a result. This vibration is illustrated in Figure 2.Ĭarbonyl stretching peaks generally fall between 19 cm -1 (assume all peak positions hereafter are in wavenumber units), a relatively unique part of the IR spectrum. Since the carbonyl group has a large dipole moment, when this group stretches and contracts the value of dµ/d x is large, thereby giving an intense peak. Additionally, remember that one of the characteristics that determines the intensity of infrared peaks is the change in dipole moment with respect to bond length, dµ/d x, during a molecular vibration (1). Recall that in a chemical bond when there are two charges separated by a distance it forms what is called a dipole moment (1). As a result, the carbonyl carbon has a large partial positive charge and the oxygen has a large partial negative charge as denoted in Figure 1. The carbon in a C=O bond is referred to as the “carbonyl carbon” as shown in Figure 1.Ĭarbonyl bonds are highly polar because of the large electronegativity difference between carbon and oxygen. The types of materials where you will find carbonyl groups include polymers, proteins, fats, solvents, and pharmaceuticals. These bonds are very common and are found in ketones, aldehydes, esters, and carboxylic acids, among others. We started with the C-H bond, moved on to the C-O bond, and now it is time to discuss the spectroscopy of the C=O or carbonyl group.
#IR SPECTRUM FUNCTIONAL GROUPS HOW TO#
In this introduction to the IR spectroscopy of the carbonyl group we explore why the peak is intense, and see how to apply that knowledge to the analysis of the spectra of ketones.Īcross all the installments I have written so far there has been an overarching structure: Each large section has been devoted to the infrared (IR) spectroscopy of a specific chemical bond. The carbonyl or C=O group is the perfect functional group for detection by infrared (IR) spectroscopy because its stretching vibration peak is intense and is located in a unique wavenumber range.


 0 kommentar(er)
0 kommentar(er)
